NEWS
2025.6
New paper in mBio
Cell wall remodeling in a fungal pathogen is required for hyphal growth into microspaces
Miki H, Gomez MM, Itani A, Yamanaka D, Sato Y, Di Pietro A, Takeshita N.

2025.6
Meeting of Japanese Society of Soil Microbiology at Tsuchiura 土壌微生物学会
Best presentation award to Takumi Kasai
2025.5
98th Annual Meeting of Japanese Society for Bacteriology at Kanazawa 細菌学会

2025.3
Fungal-bacterial biofilm collaboration paper in mBio
Aspergillus fumigatus secondary metabolite pyripyropene is important for the dual biofilm formation with Pseudomonas aeruginosa
2025.3
visited Novonesis (formerly Novozyme) in Denmark, and observed the enzyme production by fungi and recovery process.

2025.3
17th European Conference of Fungal Genetics, Dublin, Ireland

2024.11
Lab hiking to Mt. Tsukuba

2024.11
visited Academic Sinica in Taiwan and interacted with talented researchers in a wonderful research environment.
https://www.imb.sinica.edu.tw/en/index.php

2024.11
Fungal Genetics and Molecular Biology Conference at Ryukyu University in Okinawa
糸状菌分子生物学コンファレンス
Excellent presentation award to Ayaka Itani
Industry Award to Saito Kojima

2024.10
Mushroom Exhibition @ Tsukuba Experimental Botanical Garden
The true nature of mushrooms - mycelium and spores. Impressed by the excellent presentation of the materials, images and videos I provided.

2024.10
Markus Künzler from ETH Zürich gave a wonderful seminar on Insights into the defense system of a mushroom. We then enjoyed our little Oktoberfest.

2024.9
Invited to Asian Symposium of Microbial Ecology, ASME, at National Taiwan University, Taipei.
https://sites.google.com/view/asme2024-taipei/program/invited-speakers?authuser=0

2024.8
PMRN2024, Symposium of Plant Microbiota Research Network,
Japanese Society of Plant Microbe Interactions 33rd annual meeting
Poster prizes for Yuina Nomura
Symposium of Japan Society for Bioscience, Biotechnology, and Agrochemistry (JSBBA) Kanto branch
Poster prize for Hinata Miki

2024.7
New paper in PLoS Biology
Hyphae of the fungus Aspergillus nidulans demonstrate chemotropism to nutrients and pH
Yamamoto R, Miki H, Itani A, Takeshita N.
Highlights in Science, How fungi find their food
2024.6
KMB (Korean society for Microbiology and Biotechnology) and JSBBA (Japan Society for Bioscience, Biotechnology, and Agrochemistry) joint symposium at Busan, Korea.

2024.5
Plant-Microbe Interaction Workshop, Nanjing, 南京農業大学, China

2024.3
32th Fungal Genetics Conference, Asilomar, CA, USA
https://genetics-gsa.org/fungal-2024/

2023.11
Fungal Genetics and Molecular Biology Conference at Tokushima
糸状菌分子生物学コンファレンス
Industry Award for Ayaka Itani and Mahiro Toda
Japanese Society of Microbial Ecology Conference at Hamamatsu
微生物生態学会

2023.10
visited Amsterdam and Leiden and met new people and old friends

2023.9
PMRN2023, Symposium of Plant Microbiota Research Network
Poster prize for Momoka Yorinaga
Symposium of Japan Society for Bioscience, Biotechnology, and Agrochemistry (JSBBA) Kanto branch
Poster prize for Hinata Miki and Mahiro Toda
proud of you! keep going!

2023.8
Dr. Gayan, the first PhD here, has moved to Texas A&M as a postdoc.
Various memories of the 6 years. Thanks for your contributions here.
Seize the American Dream!

2023.7
Dr. Arran Hodgkinson stayed in my lab for 6 months by JSPS Postdoctoral Fellowship.
Looking forward to progress of our collaborations!

2023.3
New paper in PNAS Nexus
Local calcium signal transmission in mycelial network exhibits decentralized stress responses
Itani A, Masuo S, Yamamoto R, Serizawa T, Fukasawa Y, Takaya N, Toyota M, Betsuyaku S, Takeshita N
Press release
[Japanese]
https://www.tsukuba.ac.jp/journal/biology-environment/20230310140000.html
2023.3
EMBO | EMBL Symposium, The cellular mechanics of symbiosis, Heidelberg, Germany
https://www.embl.org/about/info/course-and-conference-office/events/ees23-01/#vf-tabs__section-speakers

2023.3
16th European Conference of Fungal Genetics, Innsbruck, Austria
https://www.ecfg16.org

2022.11
20th Fungal Genetics and Molecular Biology Conference, online
糸状菌分子生物学コンファレンス
Excellent presentation award for Miki Hinata
Industry Award for Aya Ichinose

2022.11
Japanese Society of Microbial Ecology Conference at Sapporo
Best presentation award, Gayan Abeysinghe
35th JSME conference program

2022.10
Korean Soceity of Mycology 50th anniversay internationational conference at Chonan

2022.7
Sofia and Xiaolei joined by JSPS fellowship for a few months from Greece and Germany. Enjoy life and science here!
JSPS pre-/post-doctoral fellowships for research in Japan

2022.4
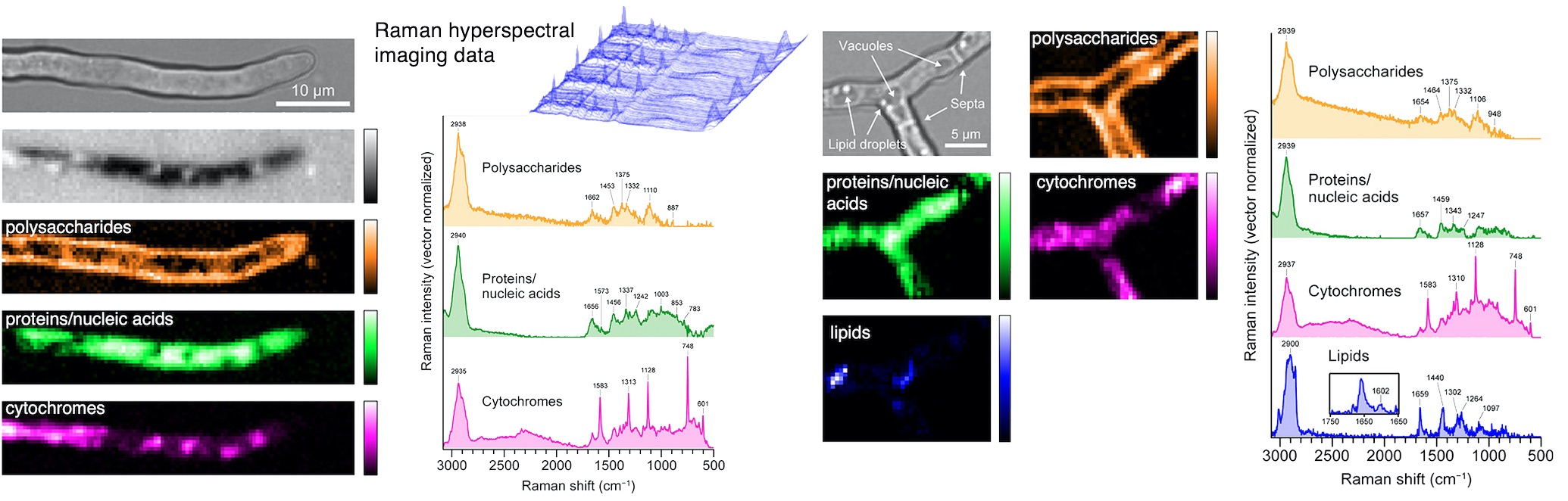
Mini-review in Microbes Environ.
Raman Micro-spectroscopy and Imaging of Filamentous Fungi.
Shigeto S, Takeshita N.
2022.3
Invited plenary session at 31th Fungal Genetics Conference, Asilomar, CA, USA
https://genetics-gsa.org/fungal-2022/invited-speakers/

2021.11
20th Fungal Genetics and Molecular Biology Conference, online
糸状菌分子生物学コンファレンス
Excellent presentation award for Riho Yamamoto
2021.10
Nomoto award, Federation of Microbiological Societies of Japan
日本微生物学連盟「野本賞」
2021.9
Tsukuba Conference 2021.
Contribution Of RIKEN BioResource Research Center To Developing Infrastructure For Life Science
2021.6
WorldMicrobeForum 2021, ASM&FEMS collaboration.
Our symposium (On-Demand). Bacterial Interactions and Symbiosis.
2021.6
Fungal Olympic Games
2021.6
Comentary by my mentor in Germany on our new mBio article performed in Japan.
Soft but Not Too Soft—How a Rigid Tube Expands without Breaking.
2021.6
The Scientist, commentary article on our new mBio article.
Fungi Squeezed Through Microchannels Offer Clues to Cell Growth
2021.3
New paper in mBio
Trade-off between Plasticity and Velocity in Mycelial Growth.
Fukuda S, Yamamoto R, Yanagisawa N, Takaya N, Sato Y, Riquelme M, Takeshita N.
Press release
https://www.tsukuba.ac.jp/en/research-news/20210316200000.html
[Japanese]
https://www.tsukuba.ac.jp/journal/biology-environment/20210316200000.html
2021.1
New paper in Sci Rep, Raman tracking of D-glucose in hyphal tips.
Deuterium-labeled Raman tracking of glucose accumulation and protein metabolic dynamics in Aspergillus nidulans hyphal tips.
Yasuda M, Takeshita N, Shigeto S.
2020.12
Cover Image, Life Science Alliance
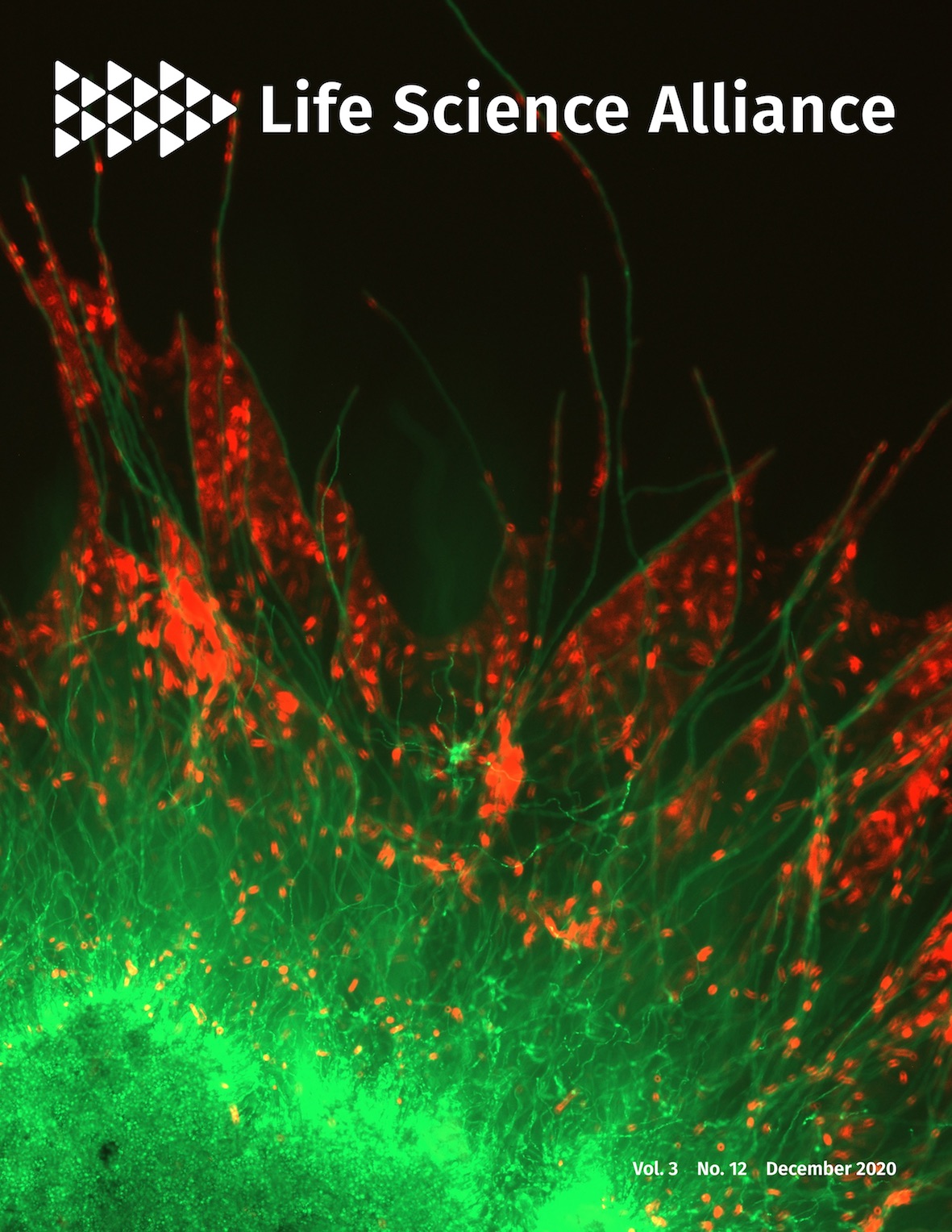
2020.11
Fungal Genetics and Molecular Biology Conference, Young workshop, online
Excellent presentation award for Riho Yamamoto
Industry Award for Shuji Hosoda
2020.10
Brewing Society of Japan Young Symposium, online
Brewing Basic Science Award for Shuji Hosoda
Sake by Research Institute of Brewing, Nomel prize
2020.9
New paper in Life Science Alliance
Fungal mycelia and bacterial thiamine establish a mutualistic growth mechanism
Abeysinghe G, Kuchira M, Kudo G, Masuo S, Ninomiya A, Takahashi K, Utada AS, Hagiwara D, Nomura N, Takaya N, Obana N, Takeshita N.
Press release
https://www.eurekalert.org/pub_releases/2020-09/uot-btb092420.php
[Japanese]
http://www.tsukuba.ac.jp/attention-research/p202009241400.html
Highlight in Science
Hyphal toll roads through the soil
2020.9
Editorial of special issue in Fungal Biology and Biotechnology
Fungal research in Japan: tradition and future
2020.6
New paper in Fungal Biology and Biotechnology
Invasive growth of Aspergillus oryzae in rice koji and increase of nuclear number.
Yasui M, Oda K, Masuo S, Hosoda S, Katayama T, Maruyama JI, Takaya N, Takeshita N.
Special issue. Fungal research in Japan: tradition and future
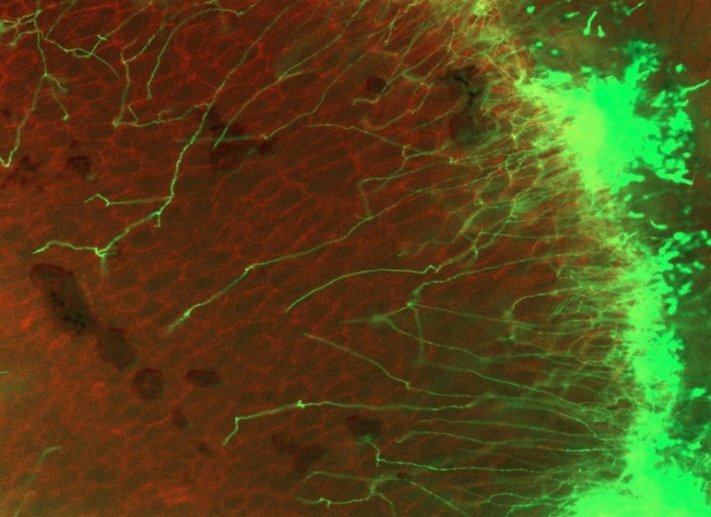
2020.4
Topics Award in JSBBA (Japan Society for Bioscience, Biotechnology and Agrochemistry) annual meeting for Sayumi Fukuda
https://jsbba.bioweb.ne.jp/jsbba2020/index.php?btn2_move=on&topics=1
2020.2
Attended to 15th European Conference on Fungal Genetics
Rome, Italy
https://www.ecfg15.org
Best Poster Award for Gayan Dakshitha at Asperfest 17.
https://www.ecfg15.org/satellite-workshops/asperfest-17/

2019.11
19th Fungal Genetics and Molecular Biology Conference
Sapporo, Japan
2019.9
New paper in Nature Communications
Comparative genomics reveals the origin of fungal hyphae and multicellularity.
Kiss E, Hegedüs B, Virágh M, Varga T, Merényi Z, Kószó T, Bálint B, Prasanna AN, Krizsán K, Kocsubé S, Riquelme M, Takeshita N, Nagy LG.
Press release, Japanese
https://www.jst.go.jp/pr/info/info1390/index.html
2019.9
New paper in Anal Chem, Fungal Cells by Raman Hyperspectral Imaging
Inhomogeneous Molecular Distributions and Cytochrome Types and Redox States in Fungal Cells Revealed by Raman Hyperspectral Imaging Using Multivariate Curve Resolution-Alternating Least Squares.
Yasuda M, Takeshita N, Shigeto S.
2019.6
Visited Karlsruhe Institute of Technology (KIT), Institut Pasteur (Paris), Gerog-August-University Goettingen, and Max Planck Institute for terrestrial microbiology (Marburg) by JST lectureship

2019.4
Topics Award in JSBBA (Japan Society for Bioscience, Biotechnology and Agrochemistry) annual meeting at Tokyo for Momoka Kuchira and Mizuki Yasui
https://jsbba.bioweb.ne.jp/jsbba2019/index.php?btn2_move=on&topics=1
2019.4
Awarded Ohsumi Frontier Science Foundation
https://www.ofsf.or.jp/activity/02_result.html
2019.3
Attended to 30th Fungal Genetics Conference
Asilomar, CA, USA
http://conferences.genetics-gsa.org/Fungal/2019/index

2018.11
18th Fungal Genetics and Molecular Biology Conference
Nagaoka, Japan
Best Poster Award for Momoka Kuchira and Sayumi Fukuda
Industry Poster Award for Mizuki Yasui
2018.9
TGSW2018, Tsukuba Global Science Week, Tsukuba
Towards Microbial Control ver. 3.0
Best Poster Award for Gayan Dakshitha and Sayumi Fukuda
Excellent Poster Award for Tomoko Serizawa
2018.9
Symposium at
82nd Annual Meeting of the Botanical Society of Japan 植物学会, Hiroshima
22nd Yeast symposium 酵母合同シンポ, Fukuoka
70th Annual meeting of the Society for Biotechnology 生物工学会, Osaka
2018.8
Gram-positive bacterium genome function conference, Atami
Excellent Poster Award for Momoka Kuchira
2018.4
New review in Microbiol Mol Biol Reviews
Fungal Morphogenesis, from the Polarized Growth of Hyphae to Complex Reproduction and Infection Structures
Riquelme M, Aguirre J, Bartnicki-García S, Braus GH, Feldbrügge M, Fleig U, Hansberg W, Herrera-Estrella A, Kämper J, Kück U, Mouriño-Pérez RR, Takeshita N, Fischer R
2018.3
Attended to 14th ECFG
Haifa, Israel
http://www.ecfg14.org

2018.1
New paper in Science Advances
Superresolution and pulse-chase imaging reveal the role of vesicle transport in polar growth of fungal cells.
Zhou L, Evangelinos M, Wernet V, Eckert A, Ishitsuka Y, Fischer R, Nienhaus GU, Takeshita N
Press release
http://www.tsukuba.ac.jp/en/research-list/p201801250910
[Japanese]
http://www.tsukuba.ac.jp/attention-research/p201801250400.html
http://www.jst.go.jp/pr/announce/20180125-2/index.html
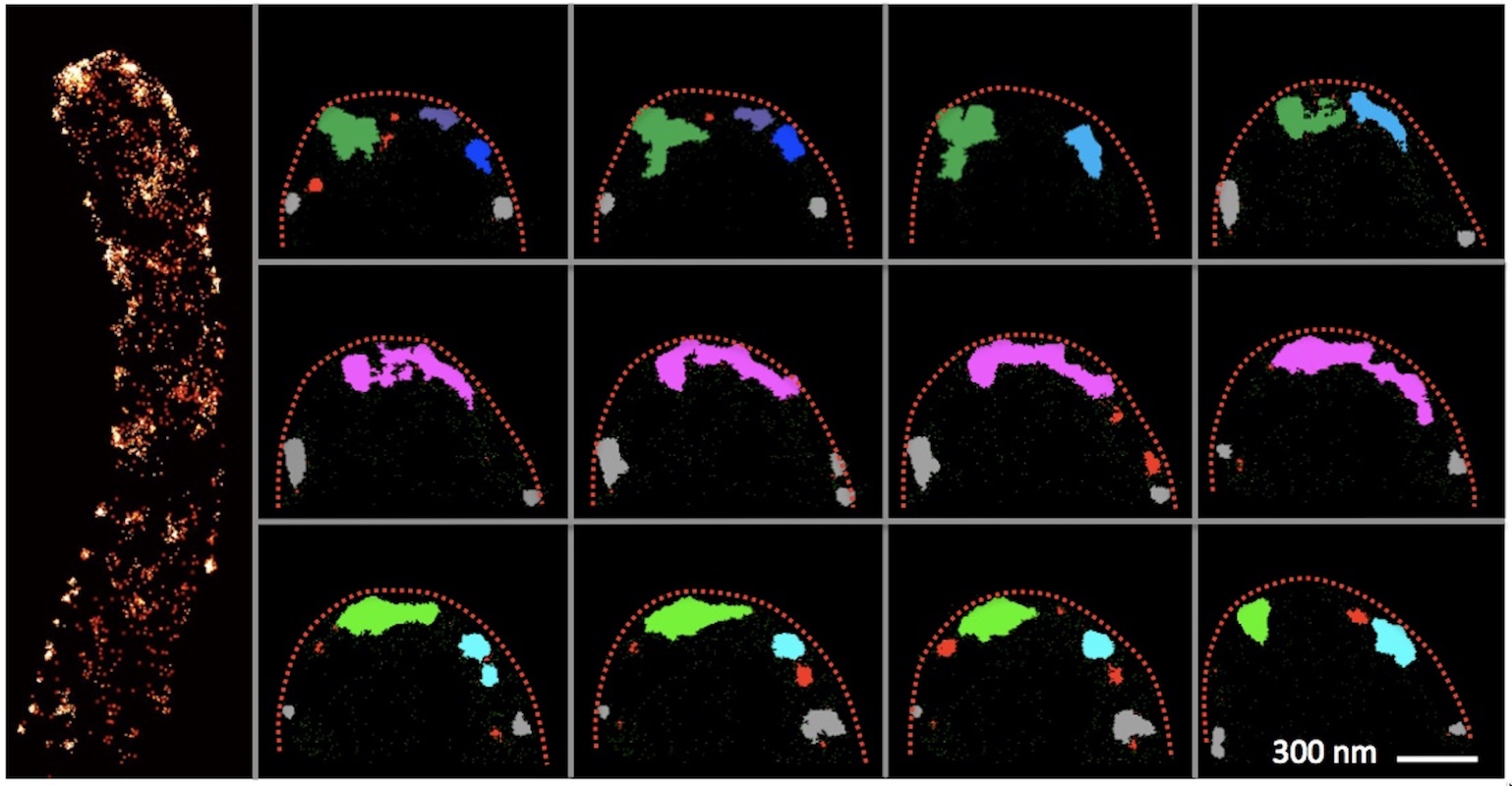
2018.1
New review in Fung Genet Biol
Oscillatory fungal cell growth.
Takeshita N
2017.12
Attended to ConBio2017
Kobe, Japan
http://www2.aeplan.co.jp/conbio2017/english/
2017.11
Attended to 17th Fungal Genetics and Molecular Biology Conference
Saga, Japan, 糸状菌分子生物学コンファレンス
http://www.biosci.osakafu-u.ac.jp/fmbsj/english/
2017.11
16th Workshop of MIcrobiology
Tokyo Institute of Technology
Poster Award; Momoka Kuchira
http://www.res.titech.ac.jp/~biores/cn20/BIKEN.html
2017.10
Gave a lecture at Max Planck Institute for Terrestrial Microbiology
Marburg, Germany

2017.10
Visited Karlsruhe for the first time in a year
Germany

2017.08
Attended to XII International Fungal Biology Conference
Incheon, South Korea
http://ifbc2017.org/register/2017_01/main.html

2017.08
New paper in Mol Microbiol
Microtubule-organizing centers of Aspergillus nidulans are anchored at septa by a disordered protein.
Zhang Y, Gao X, Manck R, Schmid M, Osmani AH, Osmani SA, Takeshita N, Fischer R.
2017.06.18
Commentary on newspaper Nikkei, 日本経済新聞
http://www.nikkei.com/article/DGKKZO17751630W7A610C1MY1000/
2017.06
Japan Society for Molecular Biology of Filamentous Fungi Award for Young Scientists, 糸状菌遺伝子研究会奨励賞
http://fungi.mysterious.jp/MAIN-J/News.html
2017. 05
Press release of the new publication in PNAS.
http://www.alphagalileo.org/ViewItem.aspx?ItemId=175296&CultureCode=en
[Japanese]
http://www.jst.go.jp/pr/announce/20170516-2/index.html
https://www.tsukuba.ac.jp/attention-research/p201705160400b.html
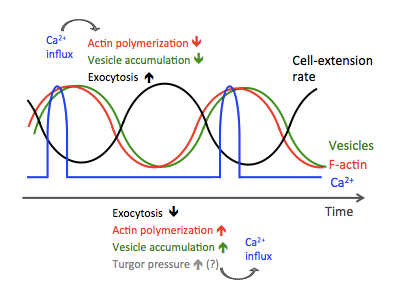
2017.03
Awarded JSBBA Award for Young Scientists
日本農芸化学会奨励賞
http://www.jsbba.or.jp/about/awards/about_awards_encouragement.html